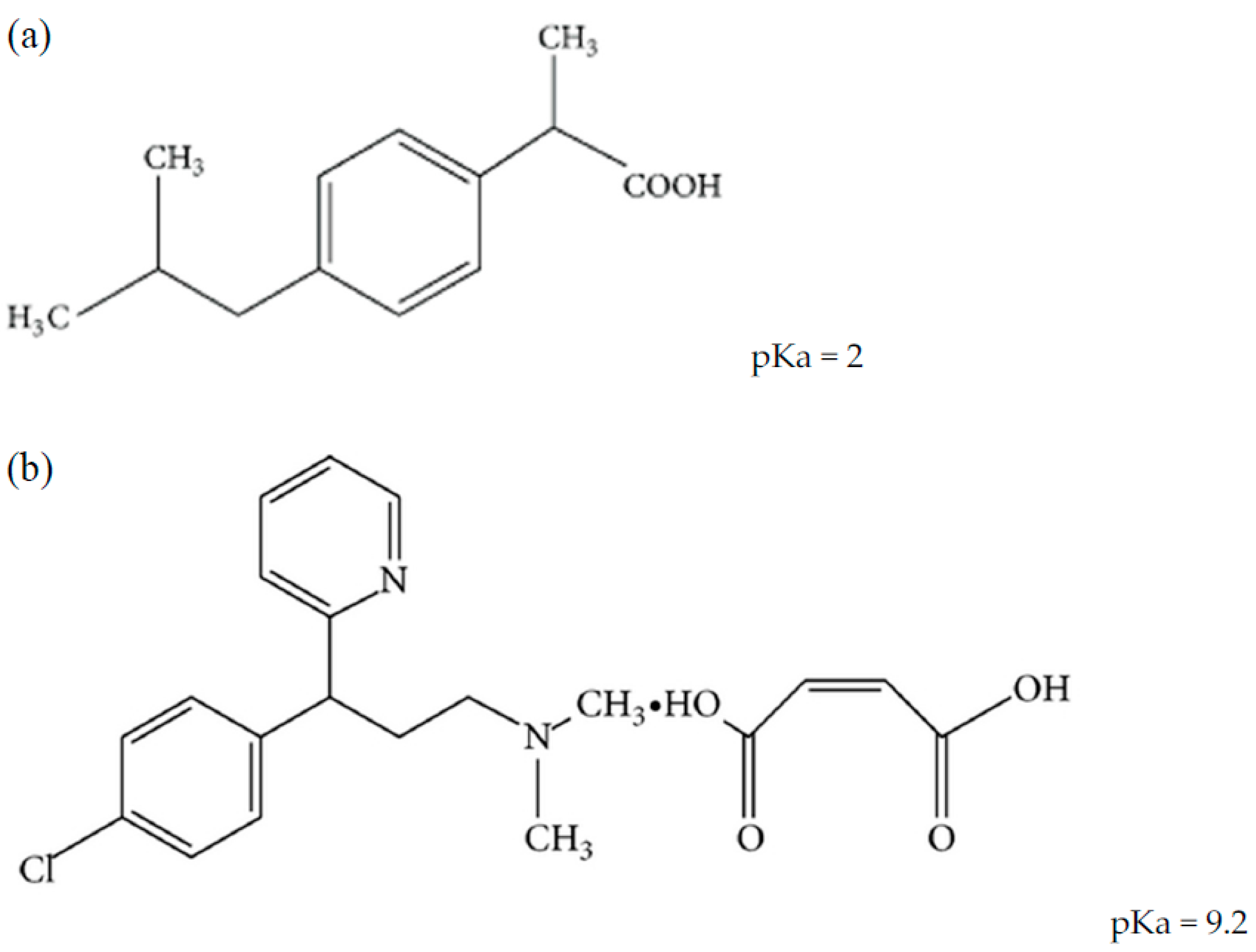
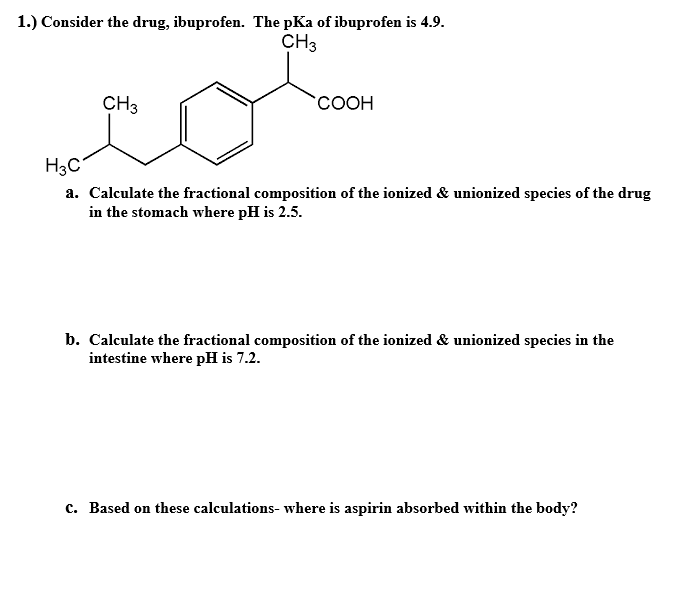
Ibuprofen: water affinity, effect of acidic pH and resonance structure:... | Download Scientific Diagram

Universal Trends between Acid Dissociation Constants in Protic and Aprotic Solvents - Busch - 2022 - Chemistry – A European Journal - Wiley Online Library
![PDF] Sorption, photodegradation, and chemical transformation of naproxen and ibuprofen in soils and water. | Semantic Scholar PDF] Sorption, photodegradation, and chemical transformation of naproxen and ibuprofen in soils and water. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/bd825006414bdf48d3e25685d998bf571ff563c7/2-Figure1-1.png)
PDF] Sorption, photodegradation, and chemical transformation of naproxen and ibuprofen in soils and water. | Semantic Scholar

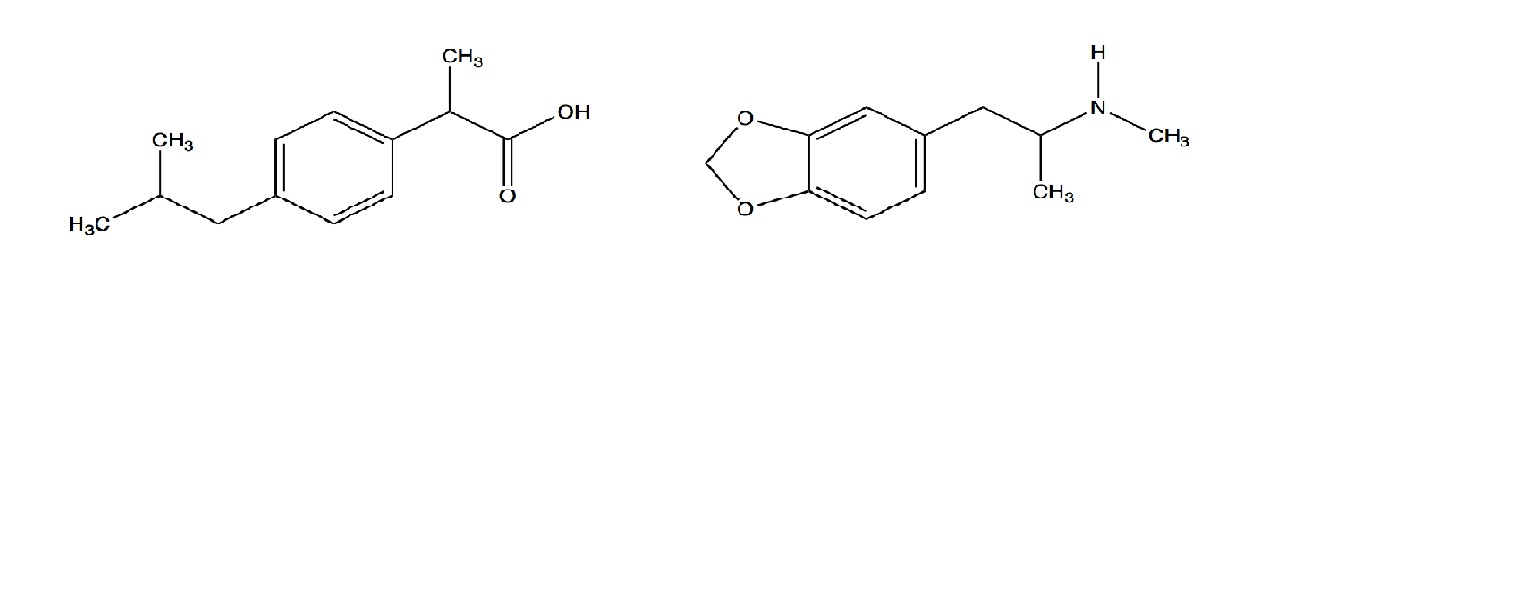
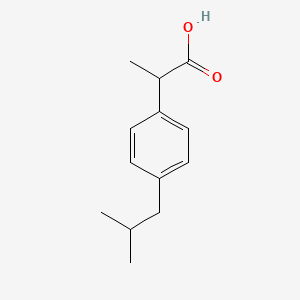
Chemical structure of ibuprofen and fenoprofen ; (*) denotes the chiral... | Download Scientific Diagram
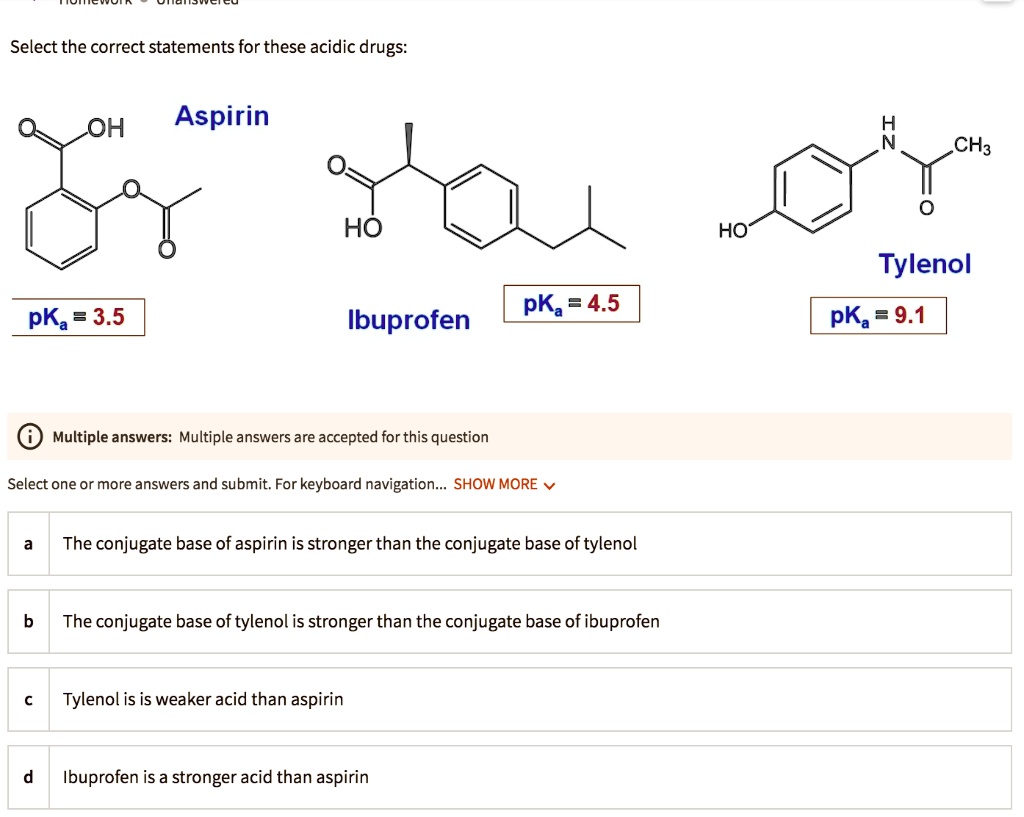
SOLVED: Select the correct statements for these acidic drugs: OH Aspirin HO HO Tylenol pKa 9.1 pKa =4.5 pKa 3.5 Ibuprofen Multiple answers: Multiple answers are accepted for this question Select one

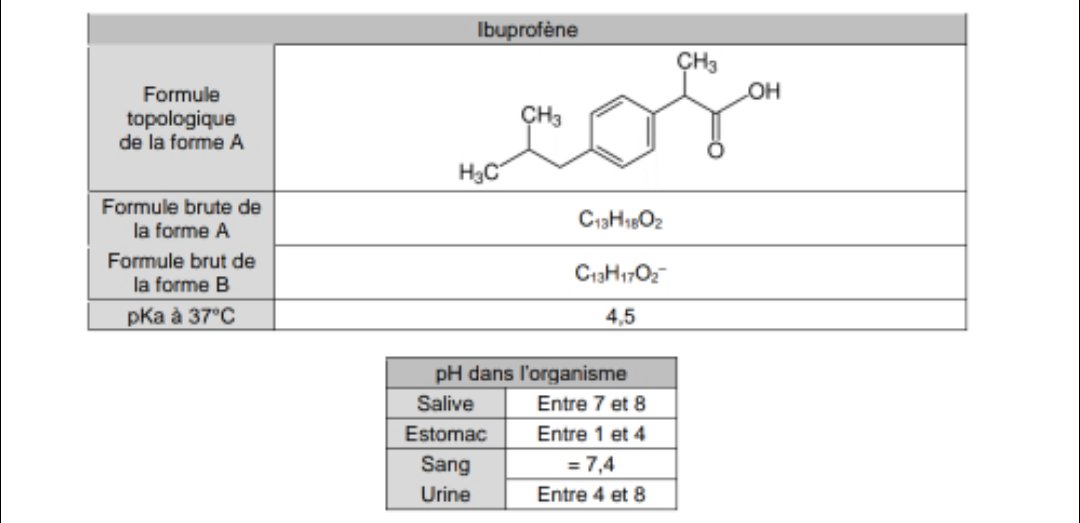
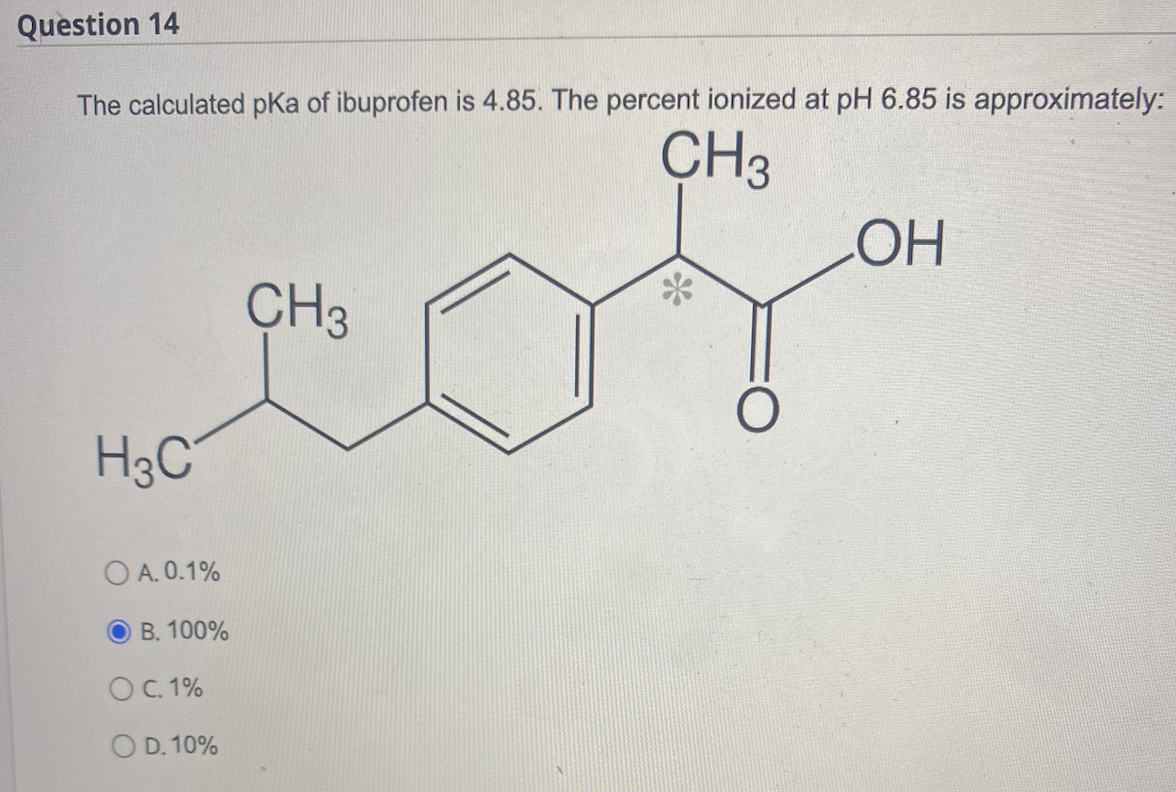
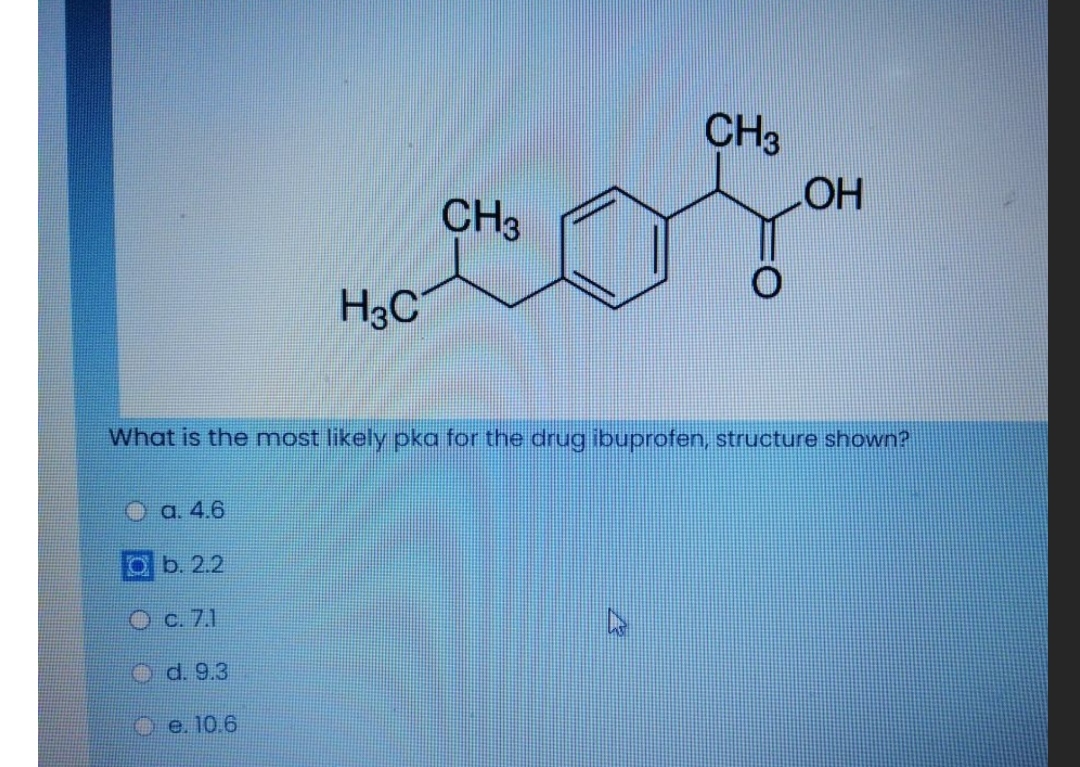
Ibuprofen (aka ADVIL) is a weak acid with a pKa of 4.9. It is absorbed through the stomach and the small - Brainly.com
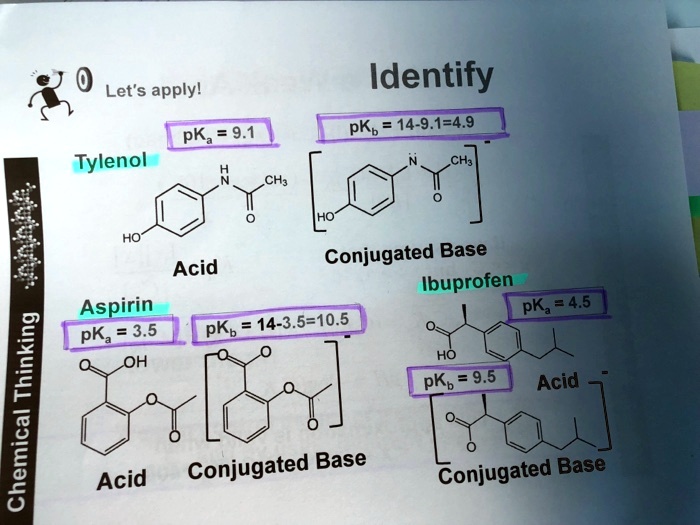
SOLVED: Let's applyl Identify pKb 14-9.1=4.9 pKa 9.1 Tylenol CHs CH; HO Conjugated Base Acid Ibuprofen Aspirin pKa =45 3.5 pKb = 14-3.5-10.5 pKa OH HO pKb 9.5 Acid 1 7 Acid Conjugated Base Conjugated Base

Potentiometric Titration Method for the Determination of Solubility Limits and pKa Values of Weak Organic Acids in Water | Analytical Chemistry

Multiple binding modes of ibuprofen in human serum albumin identified by absolute binding free energy calculations | bioRxiv
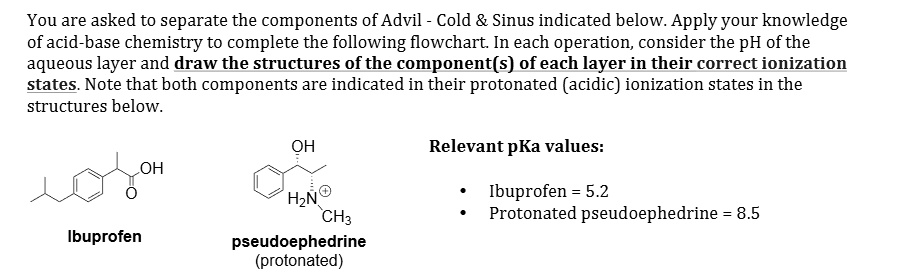
SOLVED: You are asked to separate the components of Advil Cold Sinus indicated below. Apply your knowledge of acid-base chemistry to complete the following flowchart: In each operation, consider the pH of

Comparative evaluation of ibuprofen co-crystals prepared by solvent evaporation and hot melt extrusion technology - ScienceDirect

Chemical structures and pKa values of the five different nonsteroidal... | Download Scientific Diagram